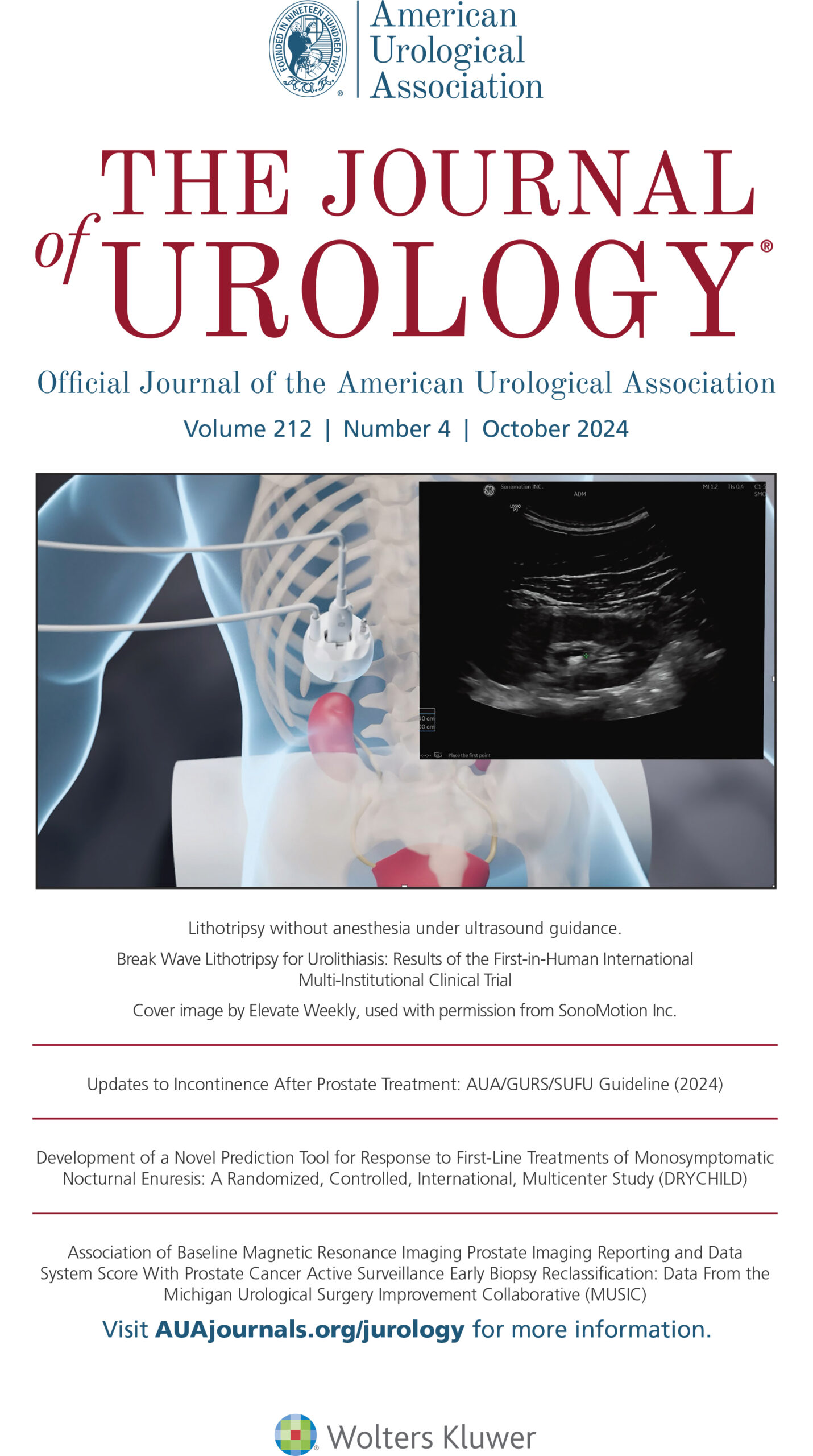
Innovations in Noninvasive Stone Treatment
SonoMotion is developing a noninvasive platform designed to fragment and reposition kidney stones at any clinical setting on fully awake patients.
Our Solution
Each year more than three million Americans see a physician for kidney stones, and over 750,000 procedures are performed to break large kidney stones. SonoMotion is developing noninvasive solutions for kidney stone disease with two innovative products called Break Wave™ and Stone Clear™.
Break Wave™
Break Wave™ therapy is designed to fragment kidney stones into small pieces, using low pressure ultrasound waves on fully awake patients, with little to no anesthesia in an office setting, in less time compared to existing options.*
Stone Clear™
Stone Clear™ therapy is designed to reposition post-lithotripsy residual fragments within a 15-minute procedure in the office on fully awake patients to help them pass their stones naturally.*
Video courtesy of University of Washington.
Over 60 Publications in Peer Reviewed Journals
First-In-Seal
First of its kind procedure successfully conducted by SonoMotion and Vancouver Aquarium on Hermes, a harbor seal suffering from kidney stones.